2023 Volume 48 Issue 2 Pages 87-97
2023 Volume 48 Issue 2 Pages 87-97
Mammalian cells generate ATP through mitochondrial respiration and glycolysis. Mitochondria not only play a key role in cell energy metabolism but also in cell cycle regulation. As a neurotoxic pollutant, benzo(a)pyrene (BaP) can trigger neuronal oxidative damage and apoptosis. However, the features of BaP-induced energy metabolism disturbance in SH-SY5Y cells has rarely been addressed. This study aimed to measure oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) as indications of respiratory activities and glycolytic. SH-SY5Y cells were treated with BaP to establish a cytotoxicity model, and butylated hydroxy anisole (BHA) was used to alleviate the damages induced by BaP. Using the Seahorse Extracellular Flux analyzer (XFp), we found that BaP significantly reduced basal respiration, ATP-linked OCR in SH-SY5Y cells with dose- and time-dependent. BHA supplementation recovered the mitochondrial respiration, synchronously attenuated intracellular ROS generation and lipid peroxidation, and simultaneously reversed the abnormal changes in antioxidant biomarkers, then rescued BaP-induced cell apoptosis. But long-term exposure to BaP or exposure to a high dosage of BaP could decrease OCR associated with maximal respiratory, spare capacity, and glycolysis metabolism. At the same time, the damage to cells is also more severe with the rate of apoptosis and mitochondrial membrane potential (ΔΨm) loss rising sharply, which were not entirely reversed by BHA. This study provides energy metabolism-related, indicative biomarkers of cytotoxicity induced by BaP, which might provide information for early prevention and intervention.
Benzo(a)pyrene (BaP), as a typical representative of polycyclic aromatic hydrocarbons (PAHs), is a ubiquitous environmental pollutant and genotoxic carcinogen (IARC, 2010; Boström et al., 2002). BaP is derived from the incomplete combustion of organic and can be found in tobacco smoke, cooked food, vehicle exhausts, and industrial emissions. Thus, people could be exposed to BaP through various routes of daily life. Owing to its lipophilicity, BaP and its metabolites can penetrate the blood-brain barrier, accumulate in different brain regions, and is hard to eliminate (Chepelev et al., 2015). Worryingly, a large number of epidemiological surveys have shown that environmental exposure to BaP correlates with a neurobehavioral and cognitive deficiency in adults, and poor neurodevelopment in children (Niu et al., 2010; Perera et al., 2015; Qiu et al., 2013; Perera et al., 2006, 2003). Rodents exposed to BaP exhibit deficits in learning and memory function and significant suppression of the hippocampus long-term potentiation (LTP) (Lyu et al., 2020). Moreover, BaP-induced neurotoxicity may occur at lower doses and at earlier times than other adverse effects (Chepelev et al., 2015; Moffat et al., 2015).
Accordingly, it is necessary to understand the precise mechanisms of BaP-induced neurodegenerative dysfunction as well as pursue precise and effective treatment strategies. Data have demonstrated that BaP-induced neuronal cell damage involved oxidative stress generation and mitochondria-mediated apoptosis (Angelova and Abramov, 2018; Chi et al., 2018; Sarma et al., 2017). Following absorption, detoxification of BaP is activated in vivo. During the detoxification of the BaP in vivo, excessive reactive oxygen species (ROS) and free radicals are produced by activated enzymes such as cytochrome P450 family 1 proteins (CYP1s) (Das et al., 2019; Chen et al., 2020). Continuously emerging data have shown that BaP significantly increased the levels of cellular ROS, including mitochondrial ROS, in a dose- or time-dependent manner (Omidian et al., 2020; Huang et al., 2021). Sun et al. (2021) reported that BaP induced mitochondrial ROS production and activated the extracellular signal-regulated kinase (ERK) pathway through AhR and CYP1s. A further study found that activation of caspase-3 is the downstream signal of the ERK pathway, which promoted apoptosis of the nerve cells (Pang et al., 2013; Li et al., 2014).
As the main source of ROS generation as well as a major target of oxidative damage, dysfunction of mitochondria has emerged as a critical event in the hippocampal neuron apoptosis (Nie et al., 2014; Wang et al., 2014). Mitochondria are the center of cellular energy metabolism, which produces energy in the form of ATP (Angelova and Abramov, 2018). And the energy demands of the brain are unusually high, which utilizes 20% of the body’s energy consumption in the normal adult human (Magistretti and Allaman, 2015). Because neuronal function critically requires a high energy metabolic demand to establish membrane excitability and to execute the complex processes of neurotransmission and plasticity (Kann and Kovács, 2007). Most studies are based on measuring ATP production to reflect mitochondrial function. Mammalian cells generate ATP through mitochondrial respiration and glycolysis. Enhanced appreciation of the role of altered energy metabolism in degenerative diseases has stimulated the development of a variety of new approaches for the assessment of mitochondrial function (Rogers et al., 2011). Actually, measurement of intact cell mitochondrial respiration and glycolysis were good indicators in evaluating the overall health of nerve cells, mainly due to the susceptibility of mitochondria to oxidative injury. However, the features of BaP-induced energy metabolism disturbance have rarely been addressed.
Butylated hydroxy anisole (BHA) is a synthetic antioxidant with applications in the chemical, pharmaceutical, and food industries (Wani and Kumar, 2015). As Liang et al. (2014) reported, BHA could prevent the harmful effects on learning and memory function in rats sub-chronically administered BaP. In addition, their study demonstrated that the activity of ATPase of rats in the BaP-BHA combination group had a significant improvement, suggesting that BHA might play a positive role in the energy metabolism (Liang et al., 2014). In the present study, human neuroblastoma SH-SY5Y cells were treated with BaP to establish a cytotoxicity model. The dose- and time-dependent effects of BaP on oxidative stress and apoptosis were analyzed, and BHA intervention was used to alleviate the damages induced by BaP. Using the Seahorse Extracellular Flux analyzer (XFp), we aimed to measure oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) as indications of respiratory activities and glycolytic. To explore the details of BaP-induced respiration metabolic and glycolysis disturbance, which provides a novel insight into the effects of BaP in damaging energy metabolism in intact cells.
BaP was purchased from Sigma (St. Louis, MO, USA). S9 Fractions (S9) was purchased from Gibco (ThermoFisher Scientific, Shanghai, China). BHA was purchased from Sangon Biotech, Shanghai, China. The Carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP), oligomycin, rotenone antimycin A, and 2-deoxyglucose (2-DG) were purchased from Sigma Aldrich, Shanghai, China. MEM / F12 medium and fetal bovine serum (FBS) were purchased from Procell Life Science & Technology, Wuhan, China. Trypsin-EDTA solution, penicillin / streptomycin mixture, and dimethyl sulfoxide (DMSO) were purchased from Solarbio Life Sciences, Beijing, China. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cell proliferation and cytotoxicity assay kit, Malondialdehyde (MDA) assay kit, and Superoxide Dismutase (SOD) typed assay kit were purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Annexin V - FITC Apoptosis Detection Kit, Reactive Oxygen Species Assay Kit, and JC-1 Apoptosis Detection Kit were purchased from Jiangsu KeyGEN BioTECH, Nanjing, China.
Cell culture and treatmentsThe human neuroblastoma SH-SY5Y cell line was obtained from Procell Life Science & Technology. SH-SY5Y cells were cultured in MEM / F12 medium containing 10% FBS and 1% penicillin / streptomycin and grown at 37°C in a cell incubator with 5% CO2. DMSO was used as a solvent for BaP; its final concentration in the culture medium did not exceed 0.1% (v / v). When the cells were grown to 80% confluence, the cells were used for the studies. For the assay, cells were treated with BaP at a final concentration of 0.1, 1.0, 10.0 μM for 24 hr, or treated with 1.0 μM BaP for 24, 48, and 72 hr, as well as with 20.0 μM BHA combination as antioxidant treatment. For metabolic activation, the final concentration of 3% S9 (v / v) was added to every culture. Cells treated only with 0.1% DMSO + 3% S9 were used as the control group.
Cells Viability assayThe MTT assay kit was used to measure cell viability quantitatively. SH-SY5Y cells were seeded at a density of 5 × 104 cells per well in 96-well assay plates in 200 µL of culture medium. 24 hr after seeding, the cells were treated with compounds at the indicated concentrations and followed the abovementioned treatment with three replicates. At the end of the treatment period, 50 µL of MTT (5 mg/mL) solution was added to each well. After incubation for 4 hr at 37°C, the medium was carefully removed, and 150 μL DMSO was added to dissolve the precipitate dye. The absorbance density values were then read by a DNM-9602G microplate reader (Beijing Perlong, Beijing, China) at 570 nm, with the cell viability expressed as a percentage of viable cells. All experiments were repeated three times independently.
Measurement of mitochondrial respiratory function and glycolysis stressThe mitochondrial respiratory function and glycolysis stress of SH-SY5Y cells after exposure to drugs was measured using a Seahorse Agilent XFp metabolic flux analyzer (Agilent Technologies, Santa Clara, CA, USA). In brief, treated and untreated cells were harvested, then 50,000 cells per well in XF assay medium were seeded onto Seahorse XF 8-well analyzer plate and allowed to attach overnight. Then cells were washed and maintained in XF Calibrant at 37°C in a non-CO2 incubator for 1 hr. During the oxygen consumption measurement assay, 1 μM oligomycin, 0.5 μM FCCP and 0.5 μM rotenone antimycin A was added to the injection ports of the XFp sensor cartridge. For glycolysis stress tests, 10 mM Glucose, 1 μM oligomycin, and 50 mM 2-DG were added to the injection ports of the XFp sensor cartridge. Finally, the OCR and the ECAR were recorded and normalized against cell numbers in each well and analyzed using the Wave software as well as the XFReport Generator (Agilent Technologies) (Plitzko and Loesgen, 2018; Zhang and Zhang, 2019).
Apoptosis assayCells were seeded (1.0 × 106 cells / well) into 6-well plates, and after BaP treatment, cells were trypsinized with 0.25% trypsin without EDTA and centrifuged at 85 g for 5 min. Apoptosis was evaluated by the annexin V - FITC apoptosis detection kit with PI to quantify apoptotic and necrotic cell death, respectively, according to the manufacturer’s protocol. Cells were resuspended in 500 μL binding buffer and incubated with 5 μL Annexin V - FITC reagent and 5 μL PI solution for 15 min at room temperature in the dark. Subsequently, the cells were analyzed using flow cytometry (FACSCanto II flow cytometer, BD Biosciences, San Jose, CA, USA).
Detection of Mitochondrial membrane potentialMitochondrial membrane potential (ΔΨm) was assessed by JC-1 (5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolcarbocyanine iodide) fluorescent cationic dye. After treatment, cells were incubated with 500 μL JC-1 solution for 20 min at 37°C. Then the fluorescence levels were analyzed by flow cytometry (FACSCanto II flow cytometer, BD Biosciences).
Intracellular ROS assayFor ROS analysis, the DCFH-DA probe and reactive oxygen species assay kit were used. The treated cells were incubated with 10 μM DCFH-DA for 30 min at 37°C. After the completed condition, the cells were washed three times. Flow cytometry analysis was performed using an excitation wavelength of 488 nm and an emission wavelength of 525 nm. The presented results are the average of at least three independent experiments.
Oxidation product assayThe cells were seeded (1.0 × 106 cells / well) in 6-well plates, then subjected to different treatment conditions. Intracellular MDA levels, which reflect oxidation levels, were quantified using the thiobarbituric acid (TBA) method, according to the manufacturer’s instructions. The absorbance at 535 nm was read using a DNM-9602G microplate reader.
Measurement of SOD activitiesSOD enzymes are considered the main defense against reactive oxygen species. Among the intracellular isoforms of SOD, copper- and zinc-containing superoxide dismutase (Cu / Zn-SOD) is widely distributed, and manganese-superoxide dismutase (Mn-SOD) exists in the mitochondria, which can both reflect the antioxidant capacity of SH-SY5Y cells. Following the manufacturer’s protocols, the activity of SOD was determined by the hydroxylamine method using a SOD typed assay kit. Cells were seeded at a density of 1.0 × 106 cells per well in 6-well plates. The activity of SOD was measured at 550 nm using a DNM-9602G microplate reader.
Statistical analysisStatistical analysis was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). All experiments were performed three times, and data are expressed as means ± standard deviation (SD). The data differences among groups were analyzed using one-way analysis of variance (ANOVA), and multiple comparisons were performed using the LSD-t test. P-value < 0.05 was considered significant statistically.
We first evaluated the cytotoxicity of BaP on SH-SY5Y cells which were detected by MTT assay at different concentrations of BaP (0, 0.1, 1.0, 10.0 μM) after 24 hr exposure. As shown in Fig. 1A, the cell viability significantly decreased in a dose-depended manner in BaP-treated cells with control cells (R2 = 0.671, F = 10.85, P < 0.001). To decipher the potential role of oxidative stress induced by BaP, the levels of ROS, MDA and the activity of SOD in SH-SY5Y cells were measured. The results indicated that the levels of ROS and MDA exhibited a progressive increase in association with the BaP exposure levels (Fig. 1B, 1C). T-SOD activity, especially Mn-SOD in cells, showed declining trends resulting from the increasing BaP concentrations (Fig. 1D). The ΔѰm and apoptosis were investigated further. The results in Fig. 1E, 1F revealed a continuous ascension in ΔѰm loss and apoptosis presented in a dose-dependent manner (R2 = 0.928, F = 27.939, P < 0.001).

BaP induced oxidative stress and apoptosis dose-dependently in human SH-SY5Y cells. SH-SY5Y cells were cultured with 0.1, 1.0 or 10.0 μM of BaP for 24 hr. (A) Cell viability was detected by MTT assay; (B) The intracellular ROS level was quantified by DCFH - DA probe by flow cytometry analysis; (C) The relative MDA levels were quantified using the TBA method; (D) The SOD activity was detected by hydroxylamine method; (E) Effect of BaP on the ΔѰ loss was assessed by JC-1 probe by flow cytometry analysis; (F) Effect of BaP on the apoptosis of SH-SY5Y cells was evaluated by the FITC / PI apoptosis detection kit by flow cytometry analysis. Data were derived from three independent experiments and presented as mean ± SD; One-Way ANOVA followed by LSD-t test for (A–F), P < 0.05 was considered significant. ***P < 0.001, **P < 0.01, *P < 0.05, compared with the control group.
To clarify the regulatory effect of BaP on the status of aerobic and anaerobic metabolism, we analyzed OCR and ECAR (Fig. 2). The steady state of OCR associated with basal respiration and ATP production was reduced by BaP in a dose-dependent manner. In the 10 μM BaP group, the proton leak, maximal respiratory and spare capacity also were attenuated compared to the control group (Fig. 2B). The glycolysis, glycolytic capacity and glycolytic reserve were damaged by 10 μM BaP (Fig. 2D). In conclusion, 10 μM BaP can decrease aerobic and anaerobic metabolism status.
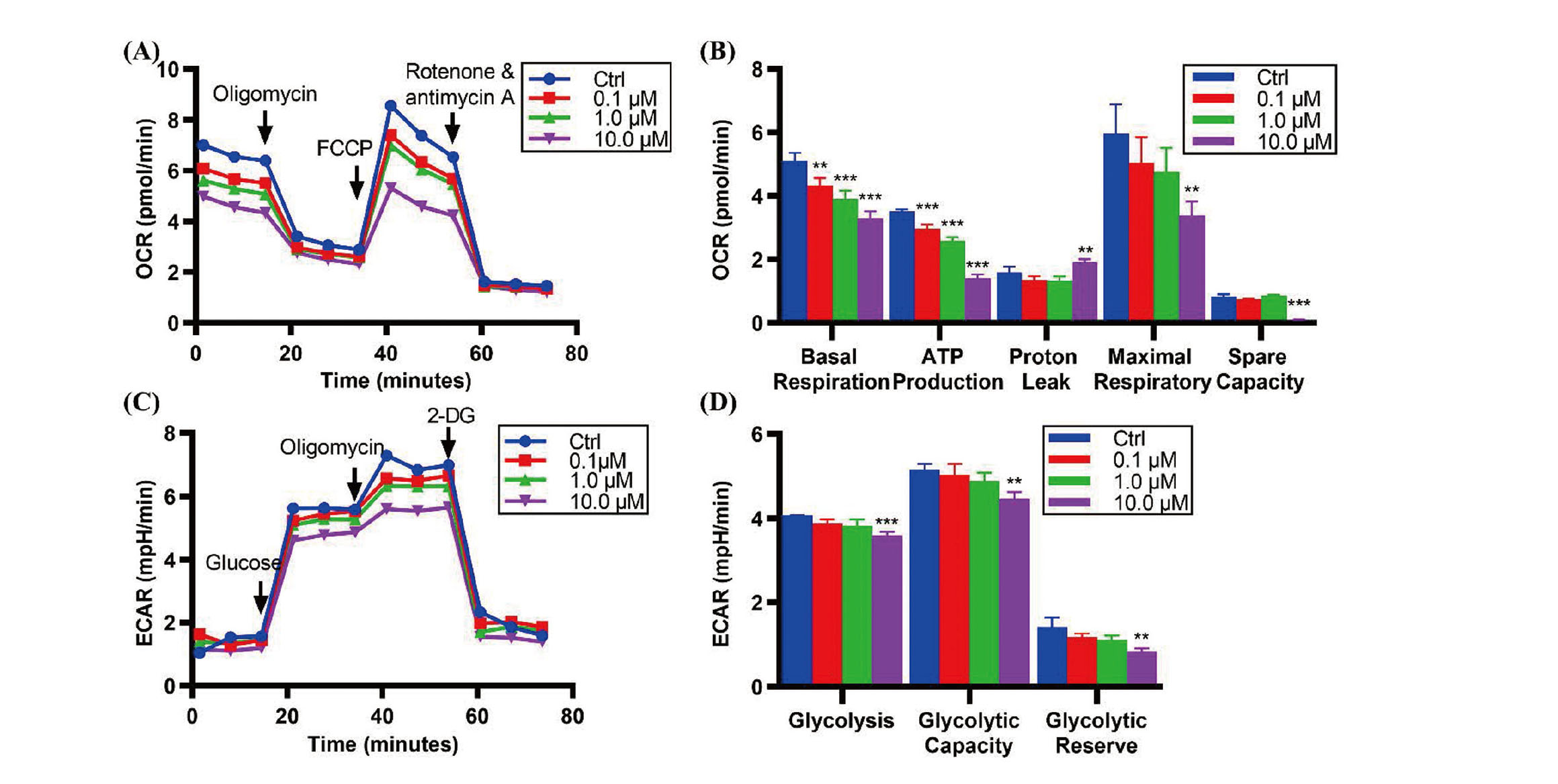
Bioenergetic profiles in SH-SY5Y cells with BaP dosages administrated. SH-SY5Y cells were untreated or treated with 0.1, 1.0 or 10.0 μM of BaP for 24 hr. (A, B) Mitochondrial respiration. The OCR was measured sequentially under basal conditions, following inhibition of ATP synthase (with 1 μM oligomycin), uncoupling the electron transporter chain (ETC) with 0.5 μM FCCP, and blocking complex I and III with 0.5 μM rotenone and antimycin A. (C, D) Glucose metabolism. After treatment, cells following 10 mM glucose, 1 μM oligomycin and 50 mM 2-DG treatments in the Glycolysis Stress assay. Data are presented as mean ± SD, n = 3 repeated experiments. One-Way ANOVA followed by LSD-t test for (A–D), P < 0.05 was considered significant. *** P < 0.001, ** P < 0.01, * P < 0.05, compared with the control group.
Then, SH-SY5Y cells were treated with 1.0 μM BaP for 24, 48, and 72 hr respectively. As shown in Fig. 3A, with the exposure time increasing, the viability of cells in BaP groups was distinctly on the decrease and fitted with a time-response relationship (R2 = 0.984, F = 198.10, P < 0.001). The intracellular ROS generation and the MDA levels significantly increased the prolongation of BaP toxicosis time (Fig. 3B, 3C). The activity of T-SOD, Cu / Zn-SOD, and Mn-SOD of SH-SY5Y cells was declining prolongation of BaP toxicosis time (Fig. 3D). The results in Fig. 3E revealed a continuous ascension in the rate of ΔΨm loss in a time-dependent manner. Similarly, the early and late-stage apoptosis rates of SH-SY5Y cells were significantly increased with a time-response relationship (R2 = 0.961, F = 111.812, P < 0.001) (Fig. 3F).

BaP induced oxidative stress and apoptosis time-dependently in human SH-SY5Y cells. SH-SY5Y cells were cultured with 1.0 μM of BaP for 24, 48 or 72 hr. (A–F) The cell viability, ROS and MDA content, SOD activity, and ΔѰ loss and apoptosis in SH-SY5Y cells were measured. Data are presented as mean ± SD, n = 3 repeated experiments. ***P < 0.001, **P < 0.01, *P < 0.05, compared with the control group.
To observe the mitochondrial respiratory function of SH-SY5Y cells as the extension of BaP exposure time, we found that the basal respiration and ATP production of cells decline significantly, and the time-effect relationship exists (Fig. 4A, 4B). The maximal respiratory and the spare capacity were decreased considerably compared to that of the control group after 48 hr of BaP exposure (Fig. 4B). Also, we could see a significant reduction in glycolysis in SH-SY5Y cells after 72 hr BaP exposure; in particular, glycolytic capacity was much worse than that at 24 hr (Fig. 4C, 4D).

Bioenergetic profiles in SH-SY5Y cells with the exposure time of BaP increased. SH-SY5Y cells were untreated or treated with 1.0 μM of BaP for 24, 48 or 72 hr, respectively. (A, B) Mitochondrial respiration. (C, D) Glucose metabolism. Data are presented as mean ± SD, n = 3 repeated experiments. ***P < 0.001, **P < 0.01, *P < 0.05, compared with the control group. ###P < 0.001, ##P < 0.01, #P < 0.05, compared with the 24 hr BaP-treated group.
To further explore the role of energy metabolism in BaP-induced oxidative stress and apoptosis, we co-administered BaP with BHA, which has been shown to protect against oxidative stress related to BaP metabolism. BHA was not toxic for SH-SY5Y cells at concentrations up to 20 μM. Further, BHA effectively alleviated 0.1 μM and 1.0 μM BaP-induced cell death (P < 0.001). In this experiment, SH-SY5Y cells were pretreated with 20 μM BHA for 1 hr before co-culturing with both BHA and 1.0 μM BaP for 24 hr, 48 hr, and 72 hr. As shown in Fig. 5A, the BHA substantially improved the cells’ viability compared to cells under the poisoning of BaP for 24 and 48 hr respectively (P < 0.001), but it was hard to save the cells treated by BaP for 72 hr. Consistently with the results of cell viability, the levels of ROS and MDA were both decreased after the employment of BHA compared to cells treated with BaP (Fig. 5B, 5C). The BHA could reverse the time-dependent reduction of the activities of SOD induced by BaP treatment (Fig. 5D). Interestingly, BHA reduced a collapse in ΔΨm from 24 hr to 72 hr treatment with BaP (Fig. 5E). Thus, after BHA utilization, apoptosis declined well compared with cells treated only with BaP. But for the 48 hr and 72 hr BaP treatment cultures, the apoptosis rate was up to 18%, which could not be entirely reversed by BHA (Fig. 5F).

BHA supplementation alleviated BaP-induced oxidative stress and apoptosis in SH-SY5Y cells. SH-SY5Y cells were pretreated with 20 μM BHA for 1 hr before being co-cultured with both BHA and 1.0 μM BaP for 24 hr, 48 hr or 72 hr. (A–F) The cell viability, ROS and MDA content, SOD activity, and ΔѰ loss and apoptosis in SH-SY5Y cells were measured. Data are presented as mean ± SD, n = 3 repeated experiments. ***P < 0.001, **P < 0.01, *P < 0.05, compared with the control group. ###P < 0.001, ##P < 0.01, #P < 0.05, compared with a single BaP-treated group.
To explore the effects of BHA on BaP-induced alteration of energy metabolism, the BHA was used to pre-treat SH-SY5Y cells. Interestingly, BHA remarkably reversed the impact of BaP-treated on basal respiration and ATP production and significantly recovered the effects of 48-72 hr BaP treatment on maximal respiratory and spare capacity (Fig. 6A–6E). No differences were found in glycolysis, maximum glycolysis, and glycolysis reserve (Fig. 6F–6H).

BHA supplementation counteracts the BaP-induced energy metabolism disturbance in SH-SY5Y cells. SH-SY5Y cells were pretreated with 20 μM BHA for 1 hr before being co-cultured with both BHA and 1.0 μM BaP for 24 hr, 48 hr or 72 hr. (A, B) Mitochondrial respiration. (C, D) Glucose metabolism. Data are presented as mean ± SD, n = 3 repeated experiments. ***P < 0.001, **P < 0.01, *P < 0.05, compared with the control group. ###P < 0.001, ##P < 0.01, #P < 0.05, compared with a single BaP-treated group.
BaP is a widespread, persistent organic pollutant. So far, many potentially adverse health effects of BaP exposure have been confirmed, and the most sensitive of the endpoints was neurological function. The association between BaP exposure and neurodegenerative disorder has been supported by substantial evidence from experimental animals and mechanistic studies (Moffat et al., 2015; Chepelev et al., 2015). Previous studies have shown that BaP induced neurotoxicity through oxidative stress and neuron death (Lin et al., 2018; Das et al., 2019; Nie et al., 2014). However, the lack of promising preventive methods calls for new research approaches that can enhance the understanding of the molecular background of BaP-induced neurotoxicity. As we know, neurons are metabolically active cells with exceptionally high and continuous energy demands. Mitochondria are the center of the cellular energy metabolism (Sanuki et al., 2017). Mitochondria are indisputably critical to neuronal function and survival (Han et al., 2021). Mitochondrial damage can cause cellular energetic status to be abnormal, and triggers oxidative stress, leakage of mitochondrial intermembranous contents into the cytosol, then causing apoptosis (Han et al., 2021). Mitochondrial dysfunction has been thought to contribute to the pathogenesis of neurodegenerative diseases (Cai and Tammineni, 2016). Mitochondria constantly change their morphology, number, and composition depending on cell type, developmental stage, environmental cues, and metabolic demands, especially in response to environmental stressors (Raftery et al., 2017). Assessments of mitochondrial bioenergetics can be used to garner insight into toxicant effects at the early stages of impairment (Souders et al., 2018). In this study, we focused on the process of energy metabolism disturbance in BaP-exposed cells in a dose- and time-dependent manner, which provided a new perspective on understanding the toxicity of BaP.
A challenge in studying the regulation of energy metabolism in neurons is the availability of appropriate cell models. Neonatal rodent primary neurons can be isolated and cultured. However, primary neurons prefer glycolysis to produce ATP rather than mitochondria (Licznerski et al., 2020; Zheng et al., 2016). Primary neurons could change their metabolism during early neuronal differentiation and synaptogenesis. We used the human neuroblastoma SH-SY5Y cell line, a simple and commonly used in vitro model related to neurotoxicity, oxidative stress, and neurodegenerative diseases (Krishna et al., 2014). Furthermore, in numerous studies, SH-SY5Y cells have been used to evaluate energy metabolism (Yang et al., 2021; Qi et al., 2022).
Firstly, a cytotoxicity model of BaP exposure was established using SH-SY5Y cells to measure the energy metabolism, oxidative stress as well as apoptosis. According to previous research, we found that the cytotoxic effect of BaP varied considerably in different cell lines, and also varied at different exposure doses. Most in vitro studies found that intracellular ROS generation, and disturbances of the activity of antioxidant enzymes can be observed under the BaP exposure concentrations from 100 nM to 100 μM (Tylichová et al., 2019; Zapletal et al., 2017; Moffat et al., 2015). Apoptosis was marked at higher concentrations of BaP (up to 2 μM) rather than somewhat at low BaP concentrations (Hockley et al., 2006; Goff et al., 2019; Kim et al., 2021; Myers et al., 2021). Supplement of antioxidant compounds, such as BHA, polyphenols, vitamin E, taurine, and atorvastatin were able to protect against BaP-induced oxidative stress and cell apoptosis (Bukowska and Duchnowicz, 2022; Omidian et al., 2017; Xu et al., 2021; Liang et al., 2014). Our findings suggest that 0.1 μM BaP induced the decline of the mitochondrial membrane potential and apoptosis of SH-SY5Y cells, and more severe with the growing concentration and time of BaP treatment. Meanwhile, supplement of BHA rescued the oxidative stress and apoptosis induced by BaP in SH-SY5Y cells. However, the changes related to mitochondrial bioenergetics in SH-SY5Y cells after BaP exposure is largely unknown. For the first study of its kind, we examined if BaP at these same concentrations or treatment time alters mitochondrial function for measuring the whole cell OCR and ECAR.
It is evident that the OCR related to basal respiration and ATP production were reduced apparently by BaP with dose increasing and time prolonging, demonstrating that BaP disturbed mitochondrial aerobic respiration. Using antioxidant BHA, we found that co-administered BHA rescued the oxidative stress and apoptosis induced at 24 hr BaP treatment. Meanwhile, the values of compromised OCR related to basal respiration and ATP production were reversed. On the other hand, the results proved that the OCR related to basal respiration and ATP production were appropriate indicators for measuring mitochondrial-dependent apoptosis. When SH-SY5Y cells were exposed to a lower dose of BaP (0.1 μM), there was no influence on OCR related to proton leak, maximum respiratory, and spare capacity. When SH-SY5Y cells were exposed to a high dose of BaP (10.0 μM) or 1.0 μM BaP up to 48 hr, the OCR associated with maximal respiratory and spare capacity declined. This suggested that the ability of SH-SY5Y cells to regulate energy metabolism balance was damaged. At this point, the apoptosis rate was more than 18%, and the levels of apoptosis could not recover entirely after BHA treatment. In the high dosage group, OCR linked to proton leak was observed. As mentioned by Souders et al. (2018) increased proton leak was considered to be related to the loss of membrane potential or integrity, which lead to impaired electron flow, then apoptosis. Previous studies have also shown that mitochondria in hippocampal neurons were lightly swelling after 10 μM BaP exposure (Jinzhu et al., 2015). We inferred that increased proton leak was the reason for more severe damage in the high dose group with the rate of apoptosis and ΔѰ loss rising sharply.
Interestingly, when SH-SY5Y cells were exposed to a lower dose of BaP, the steady state ECAR was maintained, though OCR related to basal respiration and ATP production declined. Glycolysis, glycolytic capacity and glycolytic reserve decreased when the exposure dose increased to 10.0 μM. Meanwhile, the inhibitory effect of 1.0 μM BaP prolonging the time to 72 hr on ECAR showed that more severe contrast to the exposure dose within 24 hr. Different from our current findings, Ba et al. (2015) found that 0.1 μM BaP exposure only caused a decline in the glycolysis process but enhanced the glycolytic capability in SMMC-7721 cells in vitro, indicating the long-term BaP exposure could confer SMMC-7721 cells with more robust glycolytic capability, which may benefit the cells under harsh conditions. However, with BaP treatment, no compensatory increase in glycolytic capability following inhibition of respiration in SH-SY5Y cells. There was no evidence to the idea that glycolysis could be improved following co-administered BHA.
In another study, we found that the fine particulate matter (PM2.5), which was collected in winter in the north of China, could significantly decrease mitochondrial respiration as well as glycolysis in SH-SY5Y cells with dose- and time-dependent, and further led to apoptosis (Li et al., 2022). Importantly, the application of BHA could synchronously recover the PM2.5-induced cell energy metabolism disorder. Because of a wide range of sources and highly complex components, PM2.5 is a carrier of multiple pollutants. The existing studies have analyzed the composition of PM2.5 and demonstrated that the organic components of polycyclic aromatic hydrocarbons and inorganic sulfates are essential components of PM2.5 (Cui et al., 2016). BaP is regarded as the most abundant and toxic component existing in PM2.5, which is a marker to evaluate the toxicity of PM2.5 (Fu et al., 2021; Lewandowska et al., 2018). Our current research further explained the relationship between BaP and PM2.5.
In conclusion, to gain insight into the effects of BaP exposure on energy metabolism in SH-SY5Y cells, energy metabolism, ECAR and OCR were measured as indications of glycolytic and respiratory activities. The results suggested that exposure to BaP reduced OCR related to basal respiration and ATP production. When the dose of BaP was increased or the BaP exposure time was extended, OCR associated with maximal respiratory and spare capacity began to decline, and ECAR-linked glycolysis and glycolytic capacity were damaged. The inhibition effect of BaP on OCR could be reversed by BHA, as well as by co-administration of BHA; the BaP-induced oxidative stress and apoptosis were resumed to a different degree. Altogether, this study provides energy metabolism-related, indicative biomarkers of cytotoxicity induced by BaP, which might provide information for early prevention and intervention.
We sincerely thank the Laboratory of Scientific Center of the School of Public Health, Shanxi Medical University, for assisting during the experiment.
This study was supported by the National Natural Science Foundation of China (NSFC 30872137), the Natural Science Foundation for Youths of Shanxi Province, China (20210302124301), the Key Program for International Cooperation Projects of Shanxi province (201703D421021), and a Fund for Shanxi “1331 Project” (2021-5-2-2-B1).
Conflict of interestThe authors declare that there is no conflict of interest.